There’s a method to my atomic-resolution imaging.
Aluminum is used in many applications calling for a light-weight metal. Combine aluminum with scandium, a rare-earth metal nearly as low-density as aluminum, but with a smaller diffusion rate (the rate at which molecules move through the metals, which is greatly affected by temperature), and the resulting alloy is able to withstand greater heat and has greater tensile strength. Useful for manufacturing airplanes, bicycles, firearms, and in the future, automobiles.
Does the way scandium bonds to aluminum affect alloy strength? To figure it out, researchers at Michigan Technological University use a variety of methods to understand what’s happening at the atomic level.
Paul Sanders, Patrick Horvath Endowed Associate Professor of materials science and engineering, and materials science and engineering graduate student Yang Yang, are trying to strengthen aluminum by adding scandium to it. Yang prepares the alloy samples by rapid solidification. He rapidly cools a soup of aluminum and scandium metals by dropping a small amount of liquid metal on a rotating wheel, which forces the droplet to become a ribbon as it cools to form the alloy.

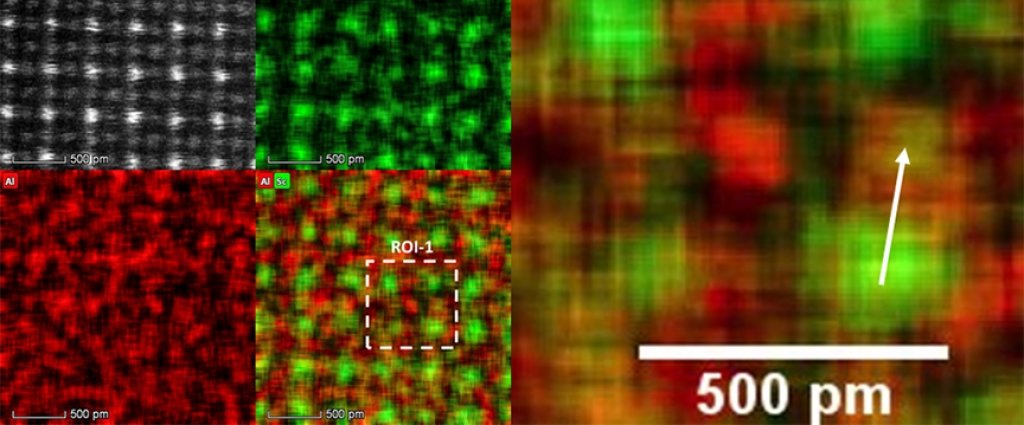
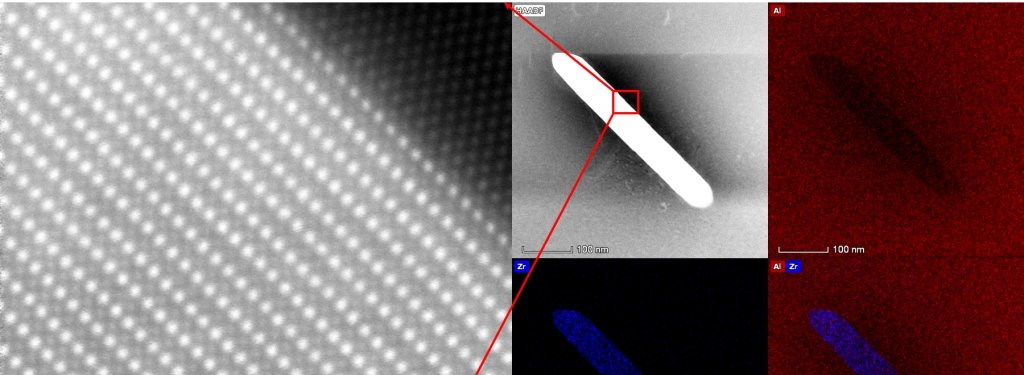
In its parent form, aluminum has a face-centered cubic crystal structure, which means the aluminum atoms are located at the corners and at the faces of a cube. Where do the scandium atoms bond with the aluminum crystal structure? Sanders and Yang used atomic resolution energy dispersive X-ray spectroscopy (EDS) mapping to determine the location of scandium atoms in the aluminum matrix.
Scientists call analytical electron microscopes “labs-in-a-machine”; in addition to imaging at atomic resolution, the microscopes provide chemical composition information of the sample. Not only can a person see how the atoms are arranged in a sample, it’s easy to determine which elements are present in it.
How does EDS work? When the high-energy electrons used for imaging in the microscopes collide with the atoms in the sample, X-rays are emitted from the sample. These X-rays carry the signatures of the elements present in the sample. The researchers use detectors to collect these characteristics X-rays.
The aberration-corrected FEI Titan Themis scanning transmission electron microscope (STEM) in Michigan Tech’s Applied Chemical and Morphological Analysis Laboratory, makes an electron beam less than an atom in width. This allows researchers to scan through samples one atom column at a time. Additionally, the lab has a SuperXTM X-ray detector, which is an array of four detectors to collect four times more X-rays than a conventional detector.
Combining the two techniques, researchers can element map at atomic resolution.
The element map shows that the scandium atoms are moving to the corner positions in a cubic crystal structure while aluminum atoms are occupying the faces of the cube. This is what’s known as a chemically ordered L12 structure. Interestingly, in one out of eight cases, researchers have found some scandium atoms in the aluminum sites. This occurs when these alloys are rapidly solidified.
These methods are powerful enough to locate a single arrested scandium atom in an aluminum site.
Michigan Technological University is an R1 public research university founded in 1885 in Houghton, and is home to nearly 7,500 students from more than 60 countries around the world. Consistently ranked among the best universities in the country for return on investment, Michigan's flagship technological university offers more than 120 undergraduate and graduate degree programs in science and technology, engineering, computing, forestry, business, health professions, humanities, mathematics, social sciences, and the arts. The rural campus is situated just miles from Lake Superior in Michigan's Upper Peninsula, offering year-round opportunities for outdoor adventure.



Comments